Containment Guidance and Tools
GAPIII and Containment Certification Scheme
The WHO Global Action Plan (GAPIII) was endorsed by the WHO Strategic Advisory Group of Experts on Immunization in October 2014 and the World Health Assembly resolution WHA68.3 in May 2015. The GAPIII Containment Certification Scheme (CCS) was endorsed by the WHO Strategic Advisory Group of Experts on Immunization in October 2016.
To access facilitor guide click here
| GAPIII | |||||||||||||||||||||
| CCS | |||||||||||||||||||||
| CCS, 1-pager |
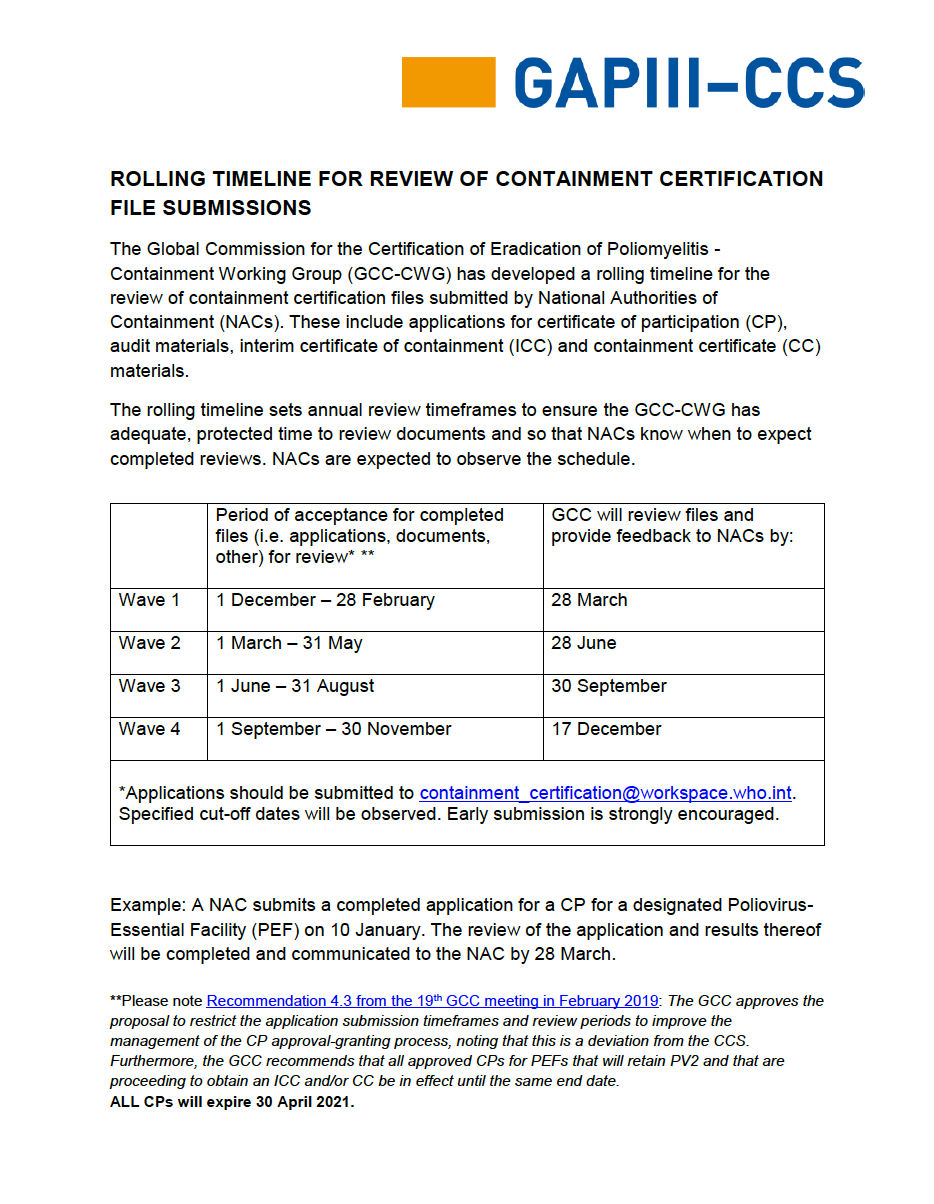
The following Containment Certification Scheme forms and templates are available to support National Authorities for Containment in the roll-out and implementation of the certification process. All submissions to the Global Certification Commission for the Eradication of Poliomyelitis (GCC) must be made in English.
Guidance to minimize risks for facilities collecting, handling or storing materials potentially infectious for polioviruses (PIM Guidance)
This guidance is intended for ALL facilities but specifically those that could be/don’t know if they are collecting, handling or storing poliovirus. These include facilities that are not purposefully using and manipulating poliovirus for research, diagnostics and or/vaccine production, but rather, might inadvertently be working with the virus through poliovirus potentially infectious material (PV PIM).
The guidance aims to help facilities identify PV PIM and eliminate or minimize risks of handling and storing such material.
| Public Health Management of Facility Related Exposure to Live Polioviruses |
Containment resolutions and scientific publications
Containment newsletters and videos
Newsletters
The Containment Corner – Poliovirus Containment News is a GPEI publication to update stakeholders on key developments in global poliovirus containment. The e-newsletter replaces Polio Pipeline.
Subscribe to receive the publication by email.
Videos
| Poliovirus Containment Animation |
| Securing a Polio-free World: Containing Polioviruses Safely and Securely |
| Looking for every last poliovirus in global sample collections |
Containment meetings and reports
Poliovirus containment in large-scale polio vaccine production and quality control facilities
This section provides updates on the demand & supply, strategies, policies and recommendations on production and quality control of polio vaccines.
- 18th WHO/UNICEF Consultation with OPV/IPV Manufacturers, National Authorities for Containment, and National Regulatory Authorities , 19 December 2019, Geneva, Switzerland
- 17th WHO/UNICEF Consultation with OPV/IPV Manufacturers and National Authorities for Containment , 9 October 2018, Geneva, Switzerland
- 16th WHO/UNICEF Consultation with OPV/IPV Manufacturers and National Authorities for Containment , 26 October 2017, Geneva, Switzerland
Meetings of Global and National Containment Certification Oversight Bodies
This section provides updates, and information on the successes and challenges of global and national containment oversight. These meetings provide a valuable opportunity for National Authorities for Containment, the Containment Working Group of the GCC and WHO to network, exchange ideas on best practices, present challenges in the implementation of national containment oversight, and discuss and identify ways forward.
- Fourth Meeting between the National Authorities for Containment and Containment Working Group for the Global Commission for Certification , 27 – 28 October 2020, virtual
- Second Face-to-Face Meeting Between the Global Certification Commission – Containment Working Group (GCC-CWG) and National Authorities for Containment (NAC) , 27 October 2018, Geneva, Switzerland
- First Face-to-Face Meeting Between the Global Certification Commission – Containment Working Group (GCC-CWG) and National Authorities for Containment (NAC) , 10-11 October 2017, Geneva, Switzerland
| PIM Guidance |
| Poliovirus Containment |
| Containment Advisory Group (CAG) and Certification Working Group (CWG) |
| Poliovirus Containment Animation |
| Poliovirus Containment Animation on YouTube |